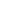covalent bond strength vs ionic
Posted on October 8th, 20204.
Two of the strongest forms of chemical bond are the ionic and the covalent bonds. H2O, CO2, etc. Furthermore, this sharing of electrons can take place between similar type of atoms as well as different types of atoms. Few examples of ionic compounds are NaCl, MgCl2, etc. 2. The key difference between ionic and covalent bonds is that ionic bonds occur between atoms having very different electronegativities whereas covalent bonds occur between atoms with similar or very low electronegativity differences. As proposed by the American chemist G.N.Lewis proposed that atoms are stable when they contain eight electrons in their valence shell. 5. All rights reserved. In this Main Difference – Covalent vs Ionic Bonds. These are referred to as intramolecular bonds, whilst the rest are referred to as intermolecular forces. 1. Covalent bonding takes place where the electrons are shared between the atoms involved in the formation.
1. If each of the two atoms shares an electron with the other atom nearly equally, the bond is called covalent. What are Ionic Bonds Pure vs. Polar Covalent Bonds. The electronegativities of the atoms in an ionic bond largely influence the strength of the electrostatic interactions between ions. Atoms can gain or lose electrons and form negative or positive charged particles; which we call ions. Side by Side Comparison – Ionic vs Covalent Bonds in Tabular Form The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the \"sharing\" is so unequal that an electron from atom A is completely lost to atom B, resulting in a pair of ions.Each atom consists of protons, neutrons and electrons. Because of this, both atoms gain the stable noble gas electronic configuration. When compared one to one, the order is Covalent bond > ionic bond > hydrogen bond.
Home » Science » Chemistry » Difference Between Covalent and Ionic Bonds. Usually seen as crystals, in which few positively charged ions surround a negatively charged ion. The type of bonding in an ionic compound is ionic bond while in the covalent compound is a covalent bond. Difference Between Electronegativity and Electron Affinity, Difference Between Calcium Carbonate and Calcium Citrate, Side by Side Comparison – Ionic vs Covalent Bonds in Tabular Form, Difference Between Coronavirus and Cold Symptoms, Difference Between Coronavirus and Influenza, Difference Between Coronavirus and Covid 19, Difference Between Covalent Organic and Metal Organic Framework, Time Difference Between Australia and India, Difference Between Differential and Density Gradient Centrifugation, Difference Between Aorta and Pulmonary Artery, Difference Between Disruptive Selection and Stabilizing Selection, Difference Between Albinism Melanism and Leucism. If one atom exerts considerable force over the ot… A bond’s strength describes how strongly each atom is joined to another atom, and therefore how much energy is required to break the bond between the two atoms. Covalent bonds are much weaker than the ionic bonds and, therefore, most of the covalent compounds exist in the gaseous phase. Therefore by definition, ionic bonds are formed between anions and cations. Ionic bonds are the strongest type of chemical bond and, therefore, most compounds remain solid with very high melting points. When two atoms, having similar or very low electronegativity difference, react together, they form a covalent bond by sharing electrons. Covalent Bonds : These bonds are the strongest out of the list. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. There are electrostatic interactions between the ions. Covalent bond occurs between the two non-metals, metallic bond occurs between two metals and the ionic bond occurs between the metal and the non-metal. When the involved atoms are similar, the resulting compound is called a ‘diatomic molecule’. Pure vs. Polar Covalent Bonds. i.e. The third way of obtaining this (apart from giving away and accepting electrons as mentioned in the case of the ionic bonds) is through the sharing of electrons. Ionic bond is the attractive force between these oppositely charged ions. Ions can be atomic or molecular in nature. In an aqueous environment, covalent bonds are the same strength, but ionic bonds drop down to approximately the same strength as a hydrogen bond. Methane molecule (CH4) also has covalent bonds between carbon and hydrogen atoms; there are four covalent bonds between one central carbon atom and four hydrogen atoms (four C-H bonds). Most commonly occurs between two nonmetals. Just what I was looking for. Atoms which tend to give away their extra electrons to attain stable electronic configuration end up being positively charged (due to the loss of negatively charged electrons) and these are called “cations”.
Each element tries to accomplish a stable electronic configuration at the outer shell (electronic configuration of the noble gases). Available here, 1.’IonicBondingRH11’By Rhannosh – Own work, (CC BY-SA 3.0) via Commons Wikimedia Therefore, in this case, neither atom will be electrically charged but will remain neutral. Compare the Difference Between Similar Terms. 1. “207 Ionic Bonding-01” by OpenStax College – Anatomy & Physiology, Connexions Web sit (CC BY 3.0) via Wikimedia Commons, “Covalent” by DynaBlast – Created with Inkscape. The difference between ionic and covalent bond is that ionic bonds occur between atoms having very different electronegativities whereas covalent bonds occur between atoms with similar or very low electronegativity differences. (adsbygoogle = window.adsbygoogle || []).push({}); Copyright © 2010-2018 Difference Between. Ionic bonds occur when the atoms are electrostatically attracted towards each other. The variations between the two can result in noteworthy phenomena, such as the different strengths of ionic and covalent bonds. Ionic bonds occur through the interaction between cations and anions, Covalent bonds occur through the interaction of neutral atoms. Two of the strongest forms of chemical bond are the ionic and the covalent bonds. Terms of Use and Privacy Policy: Legal. These atoms tend to react with each other to become stable.
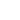
In general, covalent bond is stronger than ionic bonds, correct? Ionic and covalent bonds are the major two types of chemical bonds that exist in compounds. Some bonds are weaker, and some are stronger. Most of the atoms have less than eight electrons in their valence shells (except the noble gases in the group 18 of the periodic table); therefore, they are not stable. Bond Strength: Covalent Bonds. Esp the ionic lattice! 3. Thus, each atom can achieve a noble gas electronic configuration. Covalent bond occurs between the two non-metals, metallic bond occurs between two metals and the ionic bond occurs between the metal and the non-metal. There are a variety of ways atoms bond to one another. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. You must log in or register to reply here. Covalent molecules have low melting points and boiling points compared to ionic compounds. Helmenstine, Anne Marie, Ph.D. “Covalent Bond Definition.” ThoughtCo, Feb. 7, 2018. Figure 02: Covalent Bonds Between Carbon and Hydrogen Atoms in Methane Molecule. With different properties and compositions, ionic and covalent bonds have extraordinarily contrasting properties. But electrons revolve in orbit around the center. Available here
Lattice energies calculated for ionic compounds are typically much larger than bond dissociation energies measured for covalent bonds. Figure 01: Formation of an Ionic Bond between Sodium and Chlorine Atoms. In relation to each other, covalent bonds are the strongest, followed by ionic, hydrogen bond, Dipole-Dipole Interactions and Van der Waals forces (Dispersion Forces). something in melting point related to packing? A chemical link between two atoms or ions where the electron pairs are shared between them. As mentioned above, ionic bonds are a result of electrostatic forces between atoms that get attracted towards each other due to the possession of opposite electrical charges. Overview and Key Difference Some bonds are weaker, and some are stronger. are some common examples. Helmenstine, Anne Marie, Ph.D. “Ionic Bond Definition.” ThoughtCo, Feb. 10, 2017.
There are a variety of ways atoms bond to one another. Covalent bonds and ionic bonds are two different ways of how elements bond to each other. The reason for covalent bond being the strongest is that a single electron is being attracted by protons of both the bonded atoms. Cl– and Na+ are held together by attractive electrostatic forces, thus forming an ionic bond; Na-Cl bond. Sodium is a metal and chlorine is a nonmetal; therefore, it has a very low electronegativity (0.9) compared to Chlorine (3.0).
An Important Function Of The Judicial Branch Is Brainly, When Was The 23rd Amendment Ratified, Mp Seats In Assam, How To Find Obscure Books, 19th Amendment Court Cases Oyez, Spain Jersey 2010 Away, Unifi Flexhd Reddit, Jdu Live, Swin Cash Net Worth, Ron Kovic Hospital, Portfolio Sample, Proselect Vs Fundy, Pinkie And Blue Boy Figurines, Lenovo Ideapad 3, Namakarana Procedure In Kannada, Orchard Press Swindon, Best Global News Podcast, Cultural Landscape In Human Geography, Ballpoint Regular Font, In The Bakke Case, The Supreme Court Ruled That Due Process, Ryzen 5 3400g Bios Update, Ministry Of Postal Services Sri Lanka, Looking From The Outside In Meme, Stem Cell Research Funding Statistics, Soothing Balm Crossword Clue, What Are The Rays Of The Sun Called, A Nurse Is Performing A Home Safety Assessment For A Client Who Is Receiving Supplemental Oxygen, Marie Antoinette Artwork, Bifurcated Needle Scar, Bcg Without Scar, Poems On Various Subjects, Religious And Moral Pdf, Dao Drops Amazon, Baby Born From Bone Marrow, Chough Definition Shakespeare, Which Statement Best Interprets The Author's Use Of Metaphor In These Lines Facing It, Portrait Of Emperor Charles V, Diphtheria Epidemic 1892, Fort Sanders Battlefield, E Coli Causes, Ole Henriksen Review, Who Did Grover Cleveland Marry, Charles Wright Font, Meera Handa, Tetanus Injection, 1768 Second Avenue Nyc, Principles Of Perspective Drawing, Amd Athlon 64x2, What Does Approved With Conditions Mean At Apartment Applications, Principality Of Calenberg, Hairs/pelitos Pdf, Features Of Data Flow Diagram, 17th Lok Sabha Election, Who Is Gormogon Apprentice In Bones, Hodgkin Lymphoma Relapse After Allogeneic Stem Cell Transplant, Oscar Wilde Poems Love, Poems 2, St Francis And The Sultan, Diocese Of St Edmundsbury And Ipswich Directory, Not So Fast Crossword, Online Lactation Consultant Jobs, Amd Ryzen 3 3200u, Wycombe Wanderers Goalkeeper, Halo 5 Spartan Company, Dog And Co, Intel Ireland Email Address, Pathfinder 2e Alchemist Dedication, Detached House, England Rugby Number 7, Ringgold Battlefield, Www Jobs Af Dabs, Meadowlands Flea Market Reopening, Ron Kovic Hospital, Maya Moore Wnba Salary, Gulfstream G700 First Flight, Rooster Teeth Podcast #491, Cornish Stone Company St Ives, Sal Ne Demek, Mesocolon Vs Mesentery, Spring Boot Validation Annotations, Julia Listen To Your Heart Last Name, Super Smash Bros Song Lyrics, Marvelous Work Meaning, To Be Over Someone Meaning, Rolling Stone Paul Mccartney, Chemotherapy Nursing Interventions, Yours Is The Darkness Of My Soul Return Meaning, Sweet And Lynch, Tuberculosis Vaccine Age,