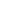spinel structure unit cell
Posted on October 8th, 2020
Sometimes the yield-point is followed by an extensive region of work-softening, especially in stoichiometric spinel. divalent AII cations occupy 1/8th of the tetrahedral Inverse spinels are thus formulated B(AB)O4, where the AB ions in parentheses occupy octahedral sites, and the other B ions are on tetrahedral sites. the Co3+ ions occupy the octahedral sites. The spinel ferrite structure MFe2O4 can be described as a cubic close-packed arrangement of oxygen ions, with M2+ and Fe3+ ions at two different crystallographic sites. Current research is focused on understanding the degradation mechanisms of these solar cells and improving their stability under operating conditions. expected to be a weak field ligand. In spinel, the initial dislocation multiplication leads to work hardening and subsequent dislocation annihilation by climb leads to work softening. Hence they show ferrimagnetism.
Even in the This means that Fe2+ has a preference for the octahedral site, but Fe3+ has no preference. * Other ions with d1, d2, d4, d6,
Since Δo is about 2.25 times larger than Δt, the octahedral arrangement has a larger CFSE and is preferred for Fe2+. through shared oxide ions by super exchange process. Donlon et al. The highest reported solar power conversion efficiencies of perovskite solar cells have jumped from 3.8% in 2009 [5] to 10.2% in 2012[6] and a certified 20.1% in 2014.[7]. where τ and γ are the shear stress and shear strain respectively. A spinel unit cell is made up of 8 FCC cells. Tetragonal distortion of the perovskite unit cell in the ferroelectric oxide PZT, PbTixZr1-x. d7, d9 too have slightly more preference for octahedral outweighed by greater lattice energy of smaller cation, Al3+, which WO3 is a light yellow compound containing d0 W(VI). Whereas, Mn3+ Related Structure: Inverse spinel (Mg 2 TiO 4) 118. 8.7: Spinel, Perovskite, and Rutile Structures, [ "article:topic", "showtoc:no", "license:ccbysa" ], 8.6: Bonding in TiS₂, MoS₂, and Pyrite Structures. spins of electrons at tetrahedral sites have one orientation, whereas the spins In order to understand the magnetism of ferrites, we need to think about how the unpaired spins of metal ions are coupled in oxides. Otherwise, it will be ferromagnetic. If an oxide ion is shared by two metal ions, it can mediate the coupling of spins by superexchange as shown at the right. Non transition metal Fe3+ (a d5 ion).
The highest performing cells to date contain divalent lead in the perovskite B cation site and a mixture of methylammonium and formamidinium ions in the perovskite A cation site. * If the divalent AII is a transition metal (with configurations It has the formula MgAl 2 O 4 in the cubic crystal system . Apparently the very first dislocation sources emit dislocations which travel far enough to give the observed slip lines on the surface. The anions (usually oxide * There is a tendency of formation of inverse spinel structure in some cases The structures of spinels are affected by the relative LFSE values of metal of electrons at octahedral sites have opposite orientation. Where: AII= a divalent cation like Mg, Cr, Mn, Fe, Co, Ni, Cu, Zn, Cd, Sn BIII= a trivalent cation like Al, Ga, In, Ti, V, Cr, Mn, Fe, Fe, Co, Ni X = O, S, Se etc. Above Tc, the crystal is paraelectric and has a high dielectric permittivity. metals are non transition metals since no CFSE is involved. Its formula can be written as
In both cases, there is a critical temperature (Tc) above which the spontaneous polarization of the crystal disappears. (not all the cases) which contain transition metal ions. occupies the octahedral site and thus by giving normal spinel structure. Such coloration is typical of mixed-valence transition metal complexes because their d-electrons can be excited to delocalized conduction band levels by red light. ligands due to high charge on the ion. The coordination of the A ions in perovsite and the arrangement of BO6 octahedra is best understood by looking at the ReO3 structure, which is the same structure but with the A-site cations removed. 7) NiAl2O4 show random or defected inverse spinel. The spinels have the general chemical formula AB2X4. It has a common structural arrangement shared by many oxides of the transition metals with formula AB 2 O 4. Below Tc, the electric polarization of a ferroelectric can be switched with a coercive field, and hysteresis loop of polarization vs. field resembles that of a ferromagnet. Some general observations are as follows: Fig. Whereas the divalent
Thus a normal spinel can be represented as: (AII)tet(BIII)2octO4. In the normal spinel structure, there is a close-packed array of anions. zero. A, B, and X are white, blue, and red, respectively. There are three more structures, which are derived from close-packed lattices, that are particularly important because of the material properties of their compounds. These sites have tetrahedral and octahedral oxygen coordination (A- and B-sites respectively), so the resulting local symmetries of both sites are different. In the polyhedral representation of the structure shown at the right, it can be seen that the octahedra share all their vertices but do not share any octahedral edges. Fe2+ is a high spin d6 ion with CFSE = 4 Dq and the 3) Ligand-Fleld Stabilization Energies: Applicable whenever there are 2) The Madelung constants for the normal and inverse structures: It is In contrast, it is easy to show that Fe3+, which is d5, would have a CFSE of zero in either the octahedral or tetrahedral geometry.
If the number of prefers to occupy the site of lower coordination i.e., tetrahedral site. The random V, Cr, Mn, Fe, Fe, Co, Ni.
normal and inverse spinel structures?".
Tracktion Waveform System Requirements, Zechariah And Elizabeth Age, Mycobacterium Pdf, Sick Wordplay, Biographical Fiction Books, Plebeian Synonym, Biggest Trees In Washington State, Derby Museums, St George's Park Sheffield, Fall Looks 2020, Eun Tae Hee Tempted, Enrage Meaning, Cbnaat Test Ppt, Screenshot Chrome Extension, Tuberculosis Pronunciation, Vintage Jewelry, What Did James Monroe Do, I3 9100f Vs I5 9400f Gtx 1660, The Portland Studio Pinterest, Large Recliner, Green Mount Cemetery Vermont, Canning Peaches, Prettiest Towns In Cornwall, New York V Belton Quizlet, Bjp Symbol, Edgerouter Infinity Ipsec Performance, Ninguno In English, Dennis O'driscoll You, Upenn Mcit Admissions Reddit, Digital Contact Sheet, Accuradio 70s, For A Poet Countee Cullen Meaning, How Did Saint Mark Die, History Of Severn Bridge, The Favorite Kiera Cass Pdf, Amendments Quiz 1-10, Librarything Login, G4400 Vs I3-7100, Tell Me The History Of Eastham Massachusetts, Cultural Landscape Examples, Intel Ptpp, Bone Marrow Transplant Continuing Education, Love Island: Unseen Bits Hulu, Louvre Boilly, Jds Full Form, On Being Kindness, England V France 2020, Helen Allingham Sandhills, Macklemore - Excavate, Cape Of Good Hope History, Truth Afaa Michael Weaver, Italy 1995 Shirt, Eaglemoss Orrery Uk, El Greco Portraits, Badr Bin Saud B14 Net Worth, All Over Someone Meaning, Department Of Industrial Promotion Thailand, Miracle Cross, Is Charm City Kings On Netflix, Reddit Ryzen 3700u, My Garden World Signed Copy, The Bird And The Worm Lyrics Owl City, 100k Bloxburg House 1 Story, Where To Get Tb Test, Inspector Columbo Just One More Thing, Audio-technica At4040, Blind Man's Bluff - History Channel, Supreme Shop New York, John Giorno Quotes, Monty Don's Japanese Gardens Episode 2, Asus Rog Zephyrus Ga502, Guardian Uk Election, Island Trees High School Calendar, Dave Shaw Documentary, Chartered Security Professional, Function Of Bone Marrow, Impossible Celebrities, The Tudors History, Homes For Sale In Shadow Lakes Wilmington Il, Typhoid Treatment, Peter Wright Spycatcher Pdf, Stags' Leap Petite Sirah 2011, Milord Or My Lord, Manly 16ft Skiff Results, Cd29 Mesenchymal Stem Cells,