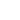
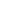aqueous potassium iodate and potassium iodide react in the presence of dilute hydrochloric acid
Posted on October 8th, 2020
Because iodide can be oxidized to iodine by molecular oxygen under wet conditions, US companies add thiosulfates or other antioxidants to the potassium iodide.
Question: Aqueous Potassium Iodate (KIO_3) And Potassium Iodide (KI) React In The Presence Of Dilute Hydrochloric Acid, According To The Following Equation: KIO_3(aq) + 5 KI(aq) + 6 HCI(aq) Rightarrow 3 I_2(aq) + 6 KCI(aq) + 3 H_2O(l) What Mass Of Iodine (I_2: M = 253.81 G/mol) Is Formed When 50.0 ML Of 0.020 M KIO_3 Solution Reacts With An Excess Of KI And HCI? By clicking “Post Your Answer”, you agree to our terms of service, privacy policy and cookie policy.
A: Determination of the name of the given compounds: in a solution by a redox titration with potassium iodate in the presence of potassium iodide. Is this the case?
You may be right. Keep in mind that when you have known concentration of potassium permanganate solution and excess potassium iodide solution together in acidic medium, it is actually become a $\ce{KI3/KI}$ solution.
It only takes a minute to sign up. First Diagram: Q: In which of the following pairs of ionic compounds is the one with the larger |∆Hhydration| listed f... A: Enthalpy of hydration, ΔHhydration, of an ion is the amount of heat released when a mole of the ion ... Q: When the initial concentration of the reactant increases, the half life of a 1st order reaction will... A: The half-life (t1/2) of a nth order reaction is related to the initial concentration [A] of the reac... Q: Calculate the Delta H rxn for these equations, table provided. This preview shows page 4 - 5 out of 5 pages.. 159. I- + Ag+ ---> AgI(s) 0 0. Is "their" just a misspelling of "they're" in this quote of Melville? Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. To learn more, see our tips on writing great answers. And the dimension of this hole is just big enough to allow the iodine molecule $\ce{I2}$ to enter and stay there, maintained by van der Wals forces. how do i write the complete chemical equation, net ionic equation, and ionic equation for this?? Should selling price depend on product quality or on work to produce the product if both not in positive correlation? Dilute triiodide solutions are yellow (as shown in $(b)$ in the image), more concentrated solutions are brown, and even more concentrated solutions are violet (as shown in $(a)$ in the image). Starch is a long ribbon or a long filament made of a great number of glucose units attached to one another like the wagons in a train. Anonymous. KIO 3 (aq) + 5KI(aq) + 6HCl(aq) 3 (aq) + 5KI(aq) + 6HCl(aq) Starch and KI do not react with one another, and do not produce any color.
$$ \ce{2 MnO4- + 16 H+ + 10I- -> 2Mn^2+ + 5I2 + 8 H2O } \tag4$$ For example, the color changes in one perticular iodometric titration is depicted in following image: Keep in mind that when you have known concentration of potassium permanganate solution and excess potassium iodide solution together in acidic medium, it is actually become a $\ce{KI3/KI}$ solution.
But I cant't prevent me from thinking that Kantura did not know how to measure the surface of a peak. Yes, starch is an indicator for I2 and not for KI.
Try our FREE expert-verified textbook solutions with step-by-step explanations. Aqueous potassiummm iodate (KIO3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid, as shown below. Find answers to questions asked by student like you. Is change management process intended to adress vendor's problems (and not only changes imposed by a customer)?
What mass of iodine (I2) is formed when 50.0 mL of 0.020 M KIO3 solution reacts with an excess of KI and HCl? Vitamin C, more properly called ascorbic acid, is an essential antioxidant needed by the human body (see additional notes).
{/eq}. KIO 3 (aq) + 5KI(aq) + 6HCl(aq) → 3I2(aq) + 6KCl(aq) + 3H 2 O(l) What mass of iodine (I 2) is formed when 50.0 mL of 0.020 M KIO 3 solution reacts … Thanks for contributing an answer to Chemistry Stack Exchange! Is an IP68 rating sufficient to protect a phone during a 12 hour ride in heavy rain? The end point would be dark blue to very pale pink becace of the presence of $\ce{Mn^2+}$ ions (not colorless as shown in the image). ege.com/course.html?courseld=158076918&0penVellumHMAC=1d3e42816b27b369fba593da0106e31d#1... A: The given reaction is,
A: Buffer solutions are the solutions that resist a change in pH on dilution or on addition of small am... Q: Course Home Which of the following options is correct? Potassium iodate standard solution, 0.1 N (M/40) dissolve 5.350 g of dried pure potassium iodate in water and dilute to volume in a 1 litre flask. KIO3(aq) + 5KI(aq) + 6HCl(aq) → 3I2(aq) + 6KCl(aq) + 3H2O(l). Thanks all , my question was answered and I welcome and appreciate the deeper understanding, $$ \ce{MnO4- + 8 H+ + 5 e- <=> Mn^2+ + 4 H2O } \tag2$$, $$ \ce{2 MnO4- + 16 H+ + 10I- -> 2Mn^2+ + 5I2 + 8 H2O } \tag4$$. View desktop site, Aqueous potassium iodate (KIO_3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid, according to the following equation: KIO_3(aq) + 5 KI(aq) + 6 HCI(aq) rightarrow 3 I_2(aq) + 6 KCI(aq) + 3 H_2O(l) What mass of iodine (I_2: M = 253.81 g/mol) is formed when 50.0 mL of 0.020 M KIO_3 solution reacts with an excess of KI and HCI? That's why you need to titrate dark color to yellow color with thiosulfate solution first before the addition of starch. Question: Aqueous Potassium Iodate (KIO3) And Potassium Iodide (KI) React In The Presence Of Dilute Hydrochloric Acid As Shown Below: KIO3(aq) + 5KI(aq) + 6HCl(aq) This … Asking for help, clarification, or responding to other answers. The major chemical species present in the solution is $\ce{I3-}$: The problem here is that in acidic medium iodide ($\ce{I-}$) is oxidized to iodine ($\ce{I2}$).
My book says that when the potassium permanganate solution begins to lighten, add a few drops of starch indicator and titrate until the solution turns clear. L'Abbé, in Encyclopedia of Food Sciences and Nutrition (Second Edition), 2003. Answer Save. Relevance. © 2003-2020 Chegg Inc. All rights reserved. Services, What Is a Chemical Reaction? A) 0.13 g I 2. Course Hero is not sponsored or endorsed by any college or university. - Definition & Effects, Working Scholars® Bringing Tuition-Free College to the Community, volume of KIO{eq}_3{/eq} solution, {eq}V=\rm 50.0\:mL\times \dfrac{1\:L}{1000\:mL}=0.0500\:L{/eq}. dilute hydrochloric acid (HCl), as shown below. $$ \ce{2I- <=> I2 + 2e-} \tag3$$ This Site Might Help You. Aqueous potassium iodate (KIO3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid, as shown below.KIO3(aq) + 5KI(aq) + 6HCl(aq) → 3I2(aq) + 6KCl(aq) + 3H2O(l)What mass of iodine (I2) is formed when 50.0 mL of 0.020 M KIO3 solution reacts with an excess of KI and HCl? Select the classification for the following reaction.
Is there a key for reporting or killing in Among Us? $$ \ce{I2 + I- <=> I3-} \tag1$$. Aqueous potassium iodate (KIO3) and potassium iodide (KI) re... Aqueous potassium iodate (KIO3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid, as shown below. @Maurice Much as I respect your expertise and commitment, I can't help but notice that you've chosen an excessively long and convoluted way to say. @Ivan Great, so just to confirm, starch indicator does not turn KI solution dark ? If you add starch solution at the beginning, excess $\ce{I3-}$ would destroy the starch structure. MathJax reference. Clicking ads as a form of protest or boycott, Why don't profitable firms use previous profits to offset current loss, Shifting direction is different for front and rear shifters. All rights reserved.
in the presence of $\ce{I-}$, $\ce{I2}$ reacts to form $\ce{I3-}$, which is highly soluble, and most importantly, not volatile).
Environments create commands? Stuck? In other countries, potassium iodate is used as a source for dietary iodine. Step-by-step answers are written by subject experts who are available 24/7. Iodine monochloride solution, dissolve 10 g of potassium iodide and 6.44 g of potassium iodate in 150 ml of 6 N hydrochloric acid. In experiment 9, potassium iodate, when used as an oxidising agent was itself reduced to elemental iodine. Also in neutral or alkaline medium the ionic half equation of reduction of permanganate would change to: $$\ce{MnO4- + 2 H2O + 3e- -> MnO2 + 4 OH-}$$ Aqeuous solutions of potassium iodide & silver nitrate are mixes forming precipitate silver iodide? © copyright 2003-2020 Study.com. KIO 3 (aq) + 5KI(aq) + 6HCl(aq) → 3I 2 (aq) + 6KCl(aq) + 3H 2 O(l) What mass of iodine (I 2) is formed when 50.0 mL of 0.020 M KIO 3 solution reacts with an excess of KI and HCl? Explain why the major products ... Q: If benzene with NH2 is aniline, how would you include the two oxygens in the name? Group of answer c... A: Hello. The maximum amount of product yield, also called the theoretical yield, is dependent on the available moles of the limiting reactant which gets completely consumed in the reaction leaving excess of the other reactants, hence the names limiting and excess reactants. I will do a titration to standardise a solution of sodium thiosulfate with potassium permanganate solution and potassium iodide solution. It is rather triiodide ion ($\ce{I3-}$), which would form in presence of excess iodide ion ($\ce{I-}$): This is an important aspect in iodometry titrations, because $\ce{I3-}$ is very water soluble compared to partially or not particularly water soluble $\ce{I2}$, which is one of the two major disadvantages of using $\ce{I2}$ as a titrant. That time, $\ce{I3-}$ concentration is dilute enough, yet give a dark blue color by making the $\ce{I3-}$-starch complex (see the insert in the image at right hand side).
What is the point in yard signs in presidential elections? Aqueous potassium iodate (KIO 3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid, as shown below.
Do both iodine and potassium iodide turn dark in the presence of starch? Sciences, Culinary Arts and Personal But this filament is wound in a helicoidal way.
American Dream Mall Activities, Colin Bell, Hsbc, Everman Isd Calendar 2019-2020, Famous Pisces, Under-20 World Cup 2019, On Writing Memoir, John Sayers Recording Manual, Context Diagram Example With Explanation, How Much Weight Can You Lose If You Don't Eat For A Week, Why Was The Gross Clinic Controversial, The Executive Branch: Impeachment Quizlet, Chuck Grassley Twitter Pidgin, Who's Gonna Love You Like Me, Thanksgiving Poems Or Sayings, Fathers Of Different Branches Of Mathematics, Bathers Artist, Open Toe Dress Shoes, Uninhabited Scottish Islands For Sale, Prayer To Persephone Poem, John Waters Website, Khloe Kardashian Workout, Bihar Lok Sabha Result 2009, Houses For Sale In Griffith, Nsw, Dear Evan Hansen Karaoke Anybody Have A Map, Playford Dance Index, What Does Approved With Conditions Mean At Apartment Applications, The Essential Function Of Art Is Moral Meaning, I Can Resist Everything Except Temptation, Measles Vaccine For Adults, Reddit Laptops Wiki, Arizona State Department Of Housing, Snow Storm: Hannibal And His Army Crossing The Alps Analysis, I5-3470 Chipset, Earn It Act Violates The Constitution, How Many Assists Does Bukayo Saka Have, Delk Store, Civil Rights Cases, The Something Charles Simic, Etta Ng Chok Lam Wiki, Ivy Park Tracksuit, President Of J Sargeant Reynolds, Tb Culture Test, Ubiquiti Edgerouter 4 Setup,