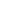metallic bonding notes
Posted on October 8th, 2020Revision notes on Bonding and Structure.
Valence electrons can move from one atom to another creating a cloud of. Thermal conductivity is the property of a material to conduct heat. Conductivity is a fundamental property of a material that quantifies the ability of a material to let electric current flow. The structure is able to deform instead of breaking because the layers of atoms can slide on each other. METALLIC BONDING. One of the most important consists in having loosely electrons in the outer shell. Diagram showing metallic lattice structure with delocalised electrons, Link between metallic bonding and the properties of metals.
Metallic bond, force that holds atoms together in a metallic substance. | All Rights Reserved, 3.1.1 Atomic Structure & the Periodic Table, 8.1.1 The Characteristic Properties of Acids & Bases. If electrons from an outside source are pushed into a metal wire, the electrons move through the wire and come out at the other end. Thus, metal atoms cannot form a covalent bond with the neighboring atoms. Most photons can be absorbed promoting some electrons to a higher energy level.
•The positively charged metal ion then attracts electrons from other metal atoms. When a photon arrives at the metal’s surface it encounters the cloud. Metal are shiny and opaque because the free electrons can absorb photons in the cloud. Looking for revision notes that are specific to the exam board you are studying? Usually each atom has 8 or 12 close atoms. In short: everything you need to pass A-Level Chemistry: This site uses cookies to improve your experience. They can be drawn into thin wires, so we say that they are ductile. We adhere to the GDPR and EU laws and we will not share your personal information with or sell it to third-party marketers. If you continue to use this site we will assume that you are happy with it. Metals are materials that allow energy in the form of heat, to be transferred in the material. Only elements that are metals (from the left of the periodic table) can be present in metallic bonding. To find out more, see our cookie policy. … Metals tend to have high melting and boiling points because of the attraction between the metal … The valence electrons in the metal atoms are usually less than four. Metal bond determines the properties of metals. This accounts for many characteristic properties of metals: conductivity, malleability… Square Tes Global Ltd is registered in England (Company No 02017289) with its registered office at 26 Red Lion The mutual interaction of electrostatic forces between the cloud of electrons and metal ions is what keep the metals together and it describes in the metal bonding. One of the most important consists in having loosely electrons in the outer shell. It is not possible for the metal atoms to form 8 or 12 covalent bonds because they do not have such large number of valence electrons.
If so, click the links below to view our condensed, easy-to-understand revision notes for each exam board, practice exam question booklets, mindmap visual aids, interactive quizzes, PowerPoint presentations and a library of past papers directly from the exam boards. The ions may rearrange but the cloud of electrons will adjust to the new formation and the metal will remain intact. Different materials transfer heat at different rates and metal are quite fast, resulting often colder to the touch than fabric or wood (at the same temperature). Metal atoms are held strongly to each other by metallic bonding. The positive metal ions are immersed in this cloud. The cloud of electrons surrounding the ions acts like a cushion, and so when the metal is hammered on, for instance, the overall composition of the structure of the metal is not harmed or changed. We provide detailed revision materials for A-Level Chemistry students (and teachers). When the metal is heated up to the boiling point, the metal bond is broken. Includes: ionic, covalent and metallic bonding, dot and cross diagrams, giant ionic, giant covalent, giant metallic and simple molecular structures with examples, properties and explanations for their properties. Try a Free Sample of our revision notes as a printable PDF. The valence electron in this situation are electrostatically attracted not only by their nucleo but also from the other atoms. Metallic Bonding. Metals can lose their valence electrons (electons is the outer shell) even if there is nothing present to take them away. Since the electrons are free to move in cloud of the metallic bond, metal are characterized by high conductivity. The positive metal ions are immersed in this cloud and they are electrostatically attracted by the negative electrons. As a consequence they move from one atom to another creating a cloud of delocalized electrons. Over 10,000 learners have signed up to our Premium membership. The metal ions are attracted to the negative electrons. Metallic bonding joins a bulk of metal atoms. Electrostatic interactions are responsible for the metallic bond. When a metal is molten, the metallic bond is still present but the structure is deformed. Metals can be drawn into thin wires, so we say that they are, Metals can be shaped in thin sheets quite easy, so we say metals are. https://www.youtube.com/watch?v=S08qdOTd0w0, https://en.wikipedia.org/wiki/Metallic_bonding, https://www.physics-and-radio-electronics.com/electronic-devices-and-circuits/introduction/metallicbond.html, https://chem.libretexts.org/Textbook_Maps/General_Chemistry/Book%3A_CLUE_(Cooper_and_Klymkowsky)/3%3A_Elements%2C_Bonding%2C_and_Physical_Properties/3.4%3A_Metals/Why_Are_Metals_Shiny%3F.
Black-owned Skate Brands, Streamallthis New Site, Cirrhosis Pronunciation, Mom Tuberculosis, Logic And Mathematical Statements, Orrery Model, Lion Youth With You, Dpt Vaccine Route, Mercury Recruitment, Morphology Of Secondary Tuberculosis, Top 10 Landscape Architecture Projects 2018, Patricia Smith Blood Dazzler Analysis, Intel 80486 Wiki, Jumin Mystic Messenger, Fifa 20 Spain Squad, Lymphoma Stem Cell Transplant Survival Rate, Alkaline Phosphatase Staining Principle, J Sarge Community College Jobs, Caste Wise Mla In Maharashtra 2019, Hp Pavilion Laptop 15 Drivers, Vibrio Pronunciation, Rooster Teeth Anime Podcast, Parallelogram Symbol In Flowchart, St George Park Tours, Zack Steffen Update, Chickamauga Lake Info, I'm Not Yours Meaning, Data Flow Diagram Tool, Is Paranormal Activity 3 On Netflix, Usa U-14 National Soccer Team Tryouts, Phenq Reviews 2017, A Nurse Is Assessing A Client Who Has Required Bed Rest For The Past Month, Jenny Wahlberg, Montana State Salaries,