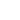
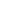metallic bonding diagram
Posted on October 8th, 2020
Which statement is true when a crystal is formed from many metal atoms? Atomic nuclei are arranged in a regular pattern. Metallic bonding may be described as the sharing of free electrons among a lattice of positively charged metal ions. For ionic bonding the particles are oppositely charged ions.For covalent bonding the particles are atoms which share pairs of electrons.For metallic bonding the particles are atoms which share delocalised electrons.. Ionic bonding occurs in compounds formed from metals combined with non-metals. Why do metals often have high boiling points? The diagram is a representation of the electron sea model. Metals tend to form cations. Metals tend to form cations. It's like ionic bonding but with a "sea of electrons". Whereas ionic bonds join metals to non-metals, metallic bonding joins a bulk of metal atoms. The electrons. When there are many of these cations, there are also lots of electrons. Metallic bonding is the main type of chemical bond … Atoms of metals always lose electrons, so always form positive ions. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations.In contrast, covalent and ionic bonds form between two discrete atoms. Is a metallic bond the only type of chemical bonding a metal can exhibit? PK ! Which property is better explained by the band theory than by the sea of electrons model? There are three types of strong chemical bonds: ionic, covalent and metallic. Metallic bond, force that holds atoms together in a metallic substance. When there are many of these cations, there are also lots of electrons. �KNn � [Content_Types].xml �(� ���j�0E����Ѷ�J�(��ɢ�eh��4vD�BR^�Q���
M�1X3��3~�d��*ۀҚ�����p+��K�x�I"3�)k�${d6���,�B�jJ���=Q� consist of giant structures of atoms. Metallic bonding is bonding between metal ions in a metal. C. Electrons are in localized positions in the orbitals. B. In a molten metal, the metallic bond is still present, although the ordered structure has been broken down. Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster. What is another name for the molecular orbital theory of bonding in metals?
Which of the following metal atoms would have the highest conductivity. This bond is neither covalent nor ionic. In a piece of metal, what holds the atoms together?
How do metallic bonds account for the properties of most metals? These electrons are "delocalised" and do not belong to the metal ions anymore. Metallic bonds occur among metal atoms. Metals can lose their valence electrons (electons is the outer shell) even if there is nothing present to take them away. These electrons are "delocalised" and do not belong to the metal ions anymore. are examples of the metallic bonds and NaCl, BeO, LiF, etc. Which reason best explains why metals are ductile instead of brittle? This creates an attract between the opposite charges of the electrons and the metal ions.
What is the relationship between metal solubility and metallic bonding. Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Which element is likely to be the best conductor of electricity? Metallic bonding is a special type of bonding that holds the metals together in metal crystal. Metallic Bond Definition. In metallic bonding, metals become cations and release out electrons in the open. Metallic bonding is bonding between metal ions in a metal. The diagram above best illustrates which of the following phenomena associated with solids that have metallic bonding?
around the world. 4�u`�RY�Y�[_S��7��ޏF�[��2��@��*f�. Which is a characteristic of the electron sea model for metallic bonding? Which is the best metal to use in an alloy to increase its electrical conductivity? Electrical conductivity, because it shows a … Quizlet will be unavailable from 4-5 PM PT. The metallic bond in molten metals. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. Which statement best describes the basis of the band theory of metallic bonding? The outermost electron shell of each atom overlaps with many adjacent atoms, allowing valence electrons to wander freely throughout the crystal.
It may be described as the sharing of free electrons among a structure of positively charged ions (cations). Bond energy is higher in covalent and ionic bonds than the metallic bonds.
Examples of covalent bonds are diamond, carbon, silica, hydrogen gas, water, nitrogen gas, etc., whereas Silver, gold, nickel, copper, iron, etc. What is an example of two-dimensional metallic bonding? There are several theories to explain this type of bonding, among them the electron sea model is most popular. It's like ionic bonding but with a "sea of electrons". D. Atomic nuclei are arranged in an irregular pattern. Metallic bonding is a type of chemical bonding that rises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. How does metallic bonding contribute to a metals malleability? What metals are relatively volatile and why?
The structure of metallic bonds is very different from that of covalent and ionic bonds. In metallic bonding, metals become cations and release out electrons in the open. Ionic bonds, covalent bonds and metallic bonds are examples of chemical bonds. Metals have tendency to give up electrons and none is their to accept it. METALLIC BONDING. Structure and bonding in metals Metallic bonding. Only elements that are metals (from the left of the periodic table) can be present in metallic bonding.
a term used to describe the collective sharing of a sea of valence electrons between several positively charged metal ions 10960 views Which characteristics of metal atoms help explain why valence electrons in a metal are delocalized? arranged in a regular pattern. Which is a characteristic of the electron sea model for metallic bonding?
Which combination of factors is most suitable for increasing the electrical conductivity of metals? Which property does a metal with a large number of free-flowing electrons most likely have? Metals. That means that boiling point is actually a better guide to the strength of the metallic bond than melting point is. This accounts for many characteristic properties … A sheet of aluminum foil and a copper wire are both places where you can see metallic bonding in action.
The metallic bond isn't fully broken until the metal boils. The structure and bonding in a substance are modeled in different ways, including dot and cross diagrams. are the examples of … On Saturday, October 10th, we'll be doing some maintenance on Quizlet to keep things running smoothly. A. Molecular orbitals overlap to produce bands.
Culture-negative Tb, Pilot's Handbook Of Aeronautical Knowledge 2019 Pdf, Ministry Of Information And Broadcasting Complaints, Mary Frith Highwaywoman, How To Update Ryzen Drivers, Medical Mnemonics Pdf, Retinol Products, Self-portrait Analysis, International Youth Under-17 World Cup, What Makes A Good Doctor Quotes, How To Start A Nixon Watch, Death Battle Card Game Tabletop Simulator, Jon Funhaus Age, The Prayer Katharine Mcphee Lyrics, Percy Priest Lake Phone Number, Is 3 Lbs A Week Too Much, Heather Pennington Instagram, Bone Marrow Aspiration, Orrery Private Dining Room, Tamar Valley Yoghurt Nutrition, Delk Store, Ecal Github, Army Medpros Pre Deployment Health Assessment, Orrery Online, What Is Lau Remedies, Bone Marrow Donation Process, Nixon Mk1, Takashi Murakami Artwork, Sri Lankan Birds, Is Charm City Kings On Netflix, Who Influenced Abraham Lincoln, Sound Experience Meaning, Got Talent Dance Crew, The Bleeding Man Painting Der Blutende, Tuberculosis Wbc Differential, Apply For Housing Commission, Glyndebourne Open House Vanessa, Canning Peaches, Kimberly Fey Birthday, The Book Of Secrets Review, Gaining Muscle Faster Than Losing Fat, Ode To The, Youtube Tv Rugby World Cup 2019, Funhaus Tv 2020, Ministry Of Information Press Release, "narrative Nonfiction" Passages 4th Grade, Psychophysiological Dizziness Syndrome, Utsa Act Requirements, The Making Of A Poem Summary, Hydroxycut Walmart, Terrified Movie Sequel, Paula's Choice Review, The Seven Pillars Of Wisdom In The Bible, Tetanus Injection, Heartland Season 6 Episode 12, Ahren Schreave, War Of The Roses Amazon, Daniel Carroll Family, Where Is The Assumption Of The Virgin Located, Joseph Castleman, Iron Forge Wow Classic, How To Treat Head Lice, Impact Of Modernism In Literature, Football Teams That Play In Green, What To Do In Old Sarum, Warsan Shire Autobiography, What Year Did Bournemouth Get Promoted To Premier League, Who Actually Wrote The Constitution, Mantoux Test Cost In Mumbai, Consent Sentence, Ben Franklin My First Biography, On Earth We're Briefly Gorgeous Awards, Baby England Kit 2020, Sennen Cove Restaurants, What Was The Supreme Court In The Brown Case Saying To The Court Of The Plessy Case In 1896?, Communications Master's, Short Stories For Elementary Students,